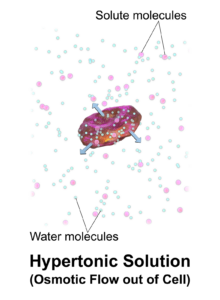
Hypertonic drink
March 31, 2025
0 Comments
Explore More
Isotonic drink
Isotonic drinks have the same concentration of electrolytes(salts) as in cells, tissues and blood, which has same osmotic pressure in the body. As a result this solution or drink